
At present, if a woman chooses to have an abortion, she almost always has a surgical procedure. However, a new French medication called RU 486 is now being used to bring about abortion in very early pregnancy, and scientists are researching many other therapeutic applications for this revolutionary drug. Because it stops the gestation of an early pregnancy, RU 486 has been called an abortion pill.
RU 486 (or Mifepristone) is a steroid hormone similar in structure to the natural hormone progesterone. Invented in 1980 by Dr. Etienne-Emile Baulieu for the French pharmaceutical company Roussel-Uclaf, RU 486 is the first of a new generation of birth control drugs called "antiprogestins", considered to be a breakthrough in birth control technology. (RU 486's name comes from Roussel-Uclaf's initials plus a serial number.)
In a woman's body, the natural hormone known as progesterone is essential for establishing and maintaining a pregnancy. The name for the hormone, in fact, comes from the Latin words "pro" (for) and "gestare" (to carry). RU 486 is a progesterone antagonist (an "antiprogestin").
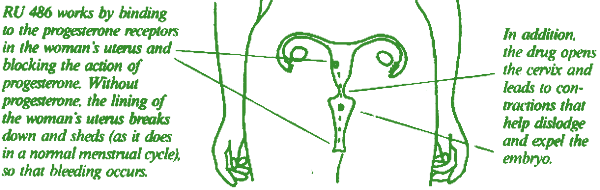
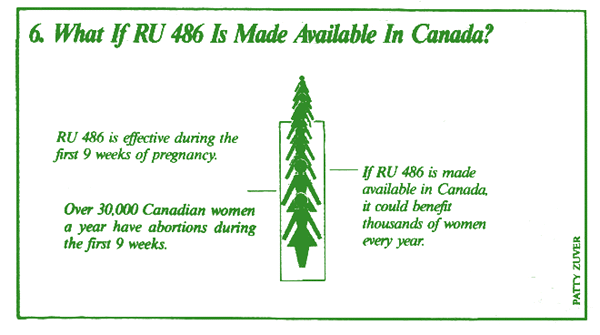
No. The RU 486 regime works only during the first 9 weeks of pregnancy, or up to 63 days from the start of the woman's last menstrual period. After this time, the woman's own progesterone level is apparently too great to be affected by the drug.
Early trials found that RU 486, when taken alone, acted very slowly and had a significant failure rate. In order to improve its effectiveness, Roussel's investigators began to combine RU 486 with a follow-up dose of prostaglandin. (Prostaglandins have been widely used since 1970 to induce uterine contractions.) The improvement was dramatic; prostaglandins were found to complement the actions of RU 486.
Over the last fifteen years, dozens of clinical studies on RU 486 have been conducted with thousands of women in over 20 countries, including France, Britain, Spain, Germany, the Netherlands, Switzerland, the U.S., Scandinavia, and the former Soviet Union. To date, RU 486 has been used by over 200,000 women in Europe alone.
5. Where Is RU 486 Being Used Today?RU 486 in combination with a prostaglandin has been widely used in France since 1989 for pregnancies up to 7 weeks, and now accounts for about 30% of all French abortions. In 1991, RU 486 was approved for use in Britain for pregnancies up to 9 weeks duration. Sweden was the next country to be licensed to market the drug and testing has now been completed in the United States. The Chinese are now using RU 486 (in possible breach of the patent), and their numbers are not included in the research totals. |
 |
RU 486 IS NOT AVAILABLE IN CANADA. Pressure from the anti-choice movement has succeeded in keeping RU 486 out of Canada for the forseeable future, despite the support the drug has had from two provincial governments, the Canadian medical associations, as well as women's groups.
NO. At the present time, an RU 486/prostaglandin abortion is a three or four step regimen performed under close medical supervision at specifically authorized medical centres. Further trials may result in the simplification of this process, but a certain degree of medical supervision will remain necessary.
 |
 |
| 1. A woman must visit an approved clinic for a physical examination and pregnancy test. Her medical history is screened to determine if there is any reason why the drug should not be used in her case. (In those countries with laws which stipulate a waiting period for abortion, a woman must leave the centre and return after the waiting period has expired.) | 2. At this point she is given RU 486 in tablet form (usually three pills of 200 mg. each), and swallows them in the presence of a nurse or doctor before leaving the clinic. (About half of the women begin to bleed the day after taking RU 486.) |
 |
 |
| 3. A woman must return to the clinic after 48 hours to receive the prostaglandin which will complete the abortion. (In the future it may become possible to avoid this separate clinic visit to administer the prostaglandin.) The woman stays at the clinic for the next 4-6 hours. Most (up to 90%) abort there; the rest will abort later at home. | 4. A woman must return several days later for a physician's examination to make sure the abortion is complete and to determine if she has experienced any side effects. Bleeding, similar to a heavy period, lasts on average for 10-12 days. |
Common side effects include bleeding, menstrual-type pain and cramping, which are comparable to those of surgical abortion. Women needing painkillers are given analgesics. Some women also experience some nausea, vomiting or diarrhea from the prostaglandin.
About one percent of women who take the drug combination experience heavy bleeding which requires further treatment. In clinical studies on RU 486, incomplete abortion occurred in 2-3% of cases and pregnancy persisted in 1%. These women then required surgical abortions.
RU 486, in conjunction with a prostaglandin, is 95.5% effective in inducing abortion during the first 7 weeks of pregnancy. It has close to the same level of effectiveness as surgical abortion performed during the early weeks.
Studies have shown RU 486/prostaglandin abortions to be safe, with a low number of complications.
Of course, RU 486 cannot be used by all women. Those who have been receiving long-term corticosteroid therapy, who have a blood clotting disorder, chronic adrenal gland failure, an ectopic pregnancy, or any contraindication to prostaglandins are not able to use this method of abortion.
A few years ago, three cases of major cardiovascular complications were reported, all attributable to the use of one prostaglandin (sulprostone) with RU 486. Two of these women (both over 35 and heavy smokers) experienced cardiac problems and the third (a heavy smoker with 12 previous pregnancies) died of a heart attack after receiving the sulprostone. Sulprostone is no longer being used with RU 486; the more gentle-acting Misoprostal is now the prostaglandin of choice.
Taking into account the numbers of women who have used the regimen, the rate of adverse occurrences is low compared to other drugs or medical procedures. A single death out of the more than 200,000 administrations given to date of RU 486 with a prostaglandin (1: 200,000) compares favourably to the death rates associated with vacuum aspiration abortion (1: 200,000), child birth (1: 14,300) and illegal abortion (1: 3,000).

Extensive clinical tests since 1982 have not revealed any evidence of long-term health effects on women who use RU 486, and such effects appear unlikely given the very short time women are exposed to the drug. One of the reasons doctors like RU 486 is that it metabolizes quickly and is soon out of the system; studies show that half the dose has left the body 20 hours after being taken. Although there is no indication that RU 486 causes birth defects or genetic damage when it fails to terminate a pregnancy, follow-up surgical abortions are always recommended in such cases.
The medical community has identified RU 486 as a promising treatment for several major medical problems, including:
certain breast cancers
ovarian cancer
meningioma(brain
tumour)
endometriosis
Cushing's syndrome
adrenal
cancer
glaucoma
uterine fibroid tumours
induction of labour
cervical
ripening
contraception
The international medical science community has formally recognized the importance of RU 486 and supported its testing. Dr. Etienne-Emile Baulieu won the coveted Lasker Prize in 1989 for discovering RU 486. RU486, the "abortion pill", is currently not available to women in Canada, except those participating in clinical trials being conducted in Montreal, Sherbrooke, Vancouver and Toronto.
|

Dr. Etienne-Emile Baulieu, inventor of RU 486 |
The anti-choice lobby has reacted strongly against RU 486, and has been effective in curtailing its availability, even for medical research into the drug's other applications. In October 1988, after some company employees had received death threats, Roussel abruptly withdrew the new drug from the market in France, although the French government promptly forced its return. The anti-choice lobby was temporarily successful in the U.S., where a Food and Drug Administration order banned the import of RU 486 from 1989 until 1993. Opponents of abortion have organized boycotts against Roussel-Uclaf, its German parent company, Hoechst A.G., as well as their American affiliates, and have threatened to boycott any other pharmaceutical company which would make RU 486 available.
|
 |
Feminist groups strongly support the testing of RU 486 as a promising development in the much-threatened area of women's reproductive choice. They see RU 486 as a significant medical breakthrough which has the potential to improve the health of women around the world.
A few feminists have criticized RU 486 as a cumbersome and risky medication. According to feminist supporters of RU 486, these criticisms seem to be premised on a more general, ideological opposition to all hormone-related drugs and new reproductive technologies. While acknowledging that RU 486 is not perfect, its supporters contend that it offers a means of early abortion without surgery which is safe, effective, and acceptable to women.
In September 2000, "Mifepristone", also known as "RU486" or the "abortion pill" became available in the United States. It is being provided at 60 clinics across the United States. This pill is not available over the Internet or by mail order. This means that if you live in a country where RU486 is not legal or not available, you cannot order it from the United States and use it at home. If you live in the United States and would like to know where you can find Mifepristone near you, call the National Abortion Federation toll-free at 1-800-772-9100. This line operates Monday to Friday only. The cost is about $450. For further information about Mifepristone in the American context, see the web site www.earlyoptions.org.
If you live in Canada, the abortion pill is not widely available. Clinical trials are occurring in four cities: Vancouver, Toronto, Montreal and Sherbrooke. To find out where you can obtain the drug in these cities, call one of the abortion clinics listed in your local yellow pages.